|
10 Chronic Diseases
linked to mitochondrial dysfunction
|
|
|

Experimental and Molecular
Pathology
|
Mitochondrial dysfunction
and
molecular pathways of disease
.
Steve R. Pieczenik, John Neustadt
Received 30 August 2006
Available online 18 January 2007
|
|
"Since the first
mitochondrial dysfunction
was described in the 1960s,
the medicine has advanced in
its understanding the role
mitochondria play in health,
disease, and aging. “
“If in
the next 50 years advances
in mitochondrial treatments
match the immense increase
in knowledge about
mitochondrial function
that has occurred in the
last 50 years,
mitochondrial diseases and
dysfunction will largely be
a medical triumph.”
“A wide range of seemingly
unrelated disorders, such
as;
schizophrenia, bipolar
disease, dementia,
Alzheimer's disease,
epilepsy, migraine
headaches, strokes,
neuropathic pain,
Parkinson's disease, ataxia,
transient ischemic attack,
cardiomyopathy, coronary
artery disease, chronic
fatigue syndrome,
fibromyalgia, retinitis
pigmentosa, diabetes,
hepatitis C, and primary
biliary cirrhosis
have underlying
pathophysiological
mechanisms in common, namely
ROS production, the
accumulation of mtDNA
damage,
resulting in
mitochondrial dysfunction.”
"Mitochondrial
dysfunction has been
implicated in nearly all
pathologic and toxicologic
conditions."
"Antioxidant
therapies hold promise for
improving mitochondrial
performance."
| |
|
|
| . |
|
What are
Mitochondria and what
functions do they
perform? |
| |
As noted in the photo below,
mitochondria are tubular or
oblong shaped and are bounded by
double membranes.
Mitochondria
play a central role in cell
life, cell death and human
health.
An increasing
number of clinical studies place
mitochondrial dysfunction at the
heart of virtually every disease
we know of.
Research also shows that many
prescribed drugs cause
mitochondrial damage.
Mitochondria are organelles that
perform a very critical role as
the
cell's electrical(energy)
producers. In
many ways,
mitochondria
act like the cells digestive
system in that they
take in nutrients and break them
down in order to create usable
energy called ATP.
This process of generating
energy within the cell is known
as cellular respiration.
If cellular respiration is low
resulting in cellular
malfunctions, it may be an
indication of some form of
mitochondrial dysfunction within
the cell rather than some
predisposed genetic defect.
Most of the chemical reactions
involved in cellular respiration
happen within the mitochondria
as they convert energy into
forms that are usable by the
cell.
The number of mitochondria in a
cell can range from a few to
several thousand, depending on
the energy requirements of each
cell. Cells known to have the
highest energy requirements and
therefore,
the highest
number of mitochondria are
muscle, heart, liver, kidney,
and brain cells.
|
|
|
|
|
|
Genetics
vs
Metabolics
|
|
|
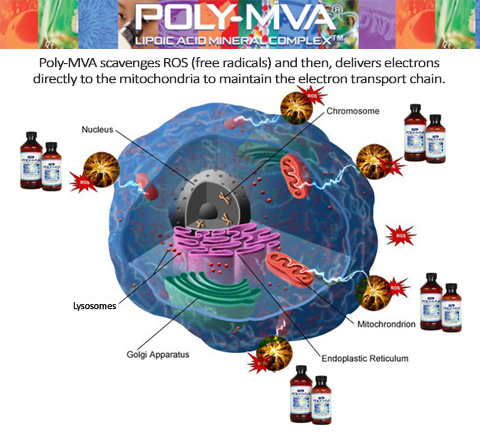
Poly-MVA
formulation
scavenge
free
radicals(ROS)
while transporting
Electrons(Energy),
Vitamins, Minerals
and
ATP
Co-Factors
directly to mitochondria.
|
|
Over 50 years ago,
most
scientific and medical
therapeutic
approaches focused
on cellular metabolism.
.
With the advent of genetics, a
concentrated shift toward
genomics, and subsequently
proteomics (protein profiles),
dominated the therapeutic stage.
.
The area of metabolism
(metabolomics)
is now
being revisited as an
attractive target.
One such regulatory approach is
via the
manipulation of
cellular energy.
Cellular energy is
synonymous with metabolic power.
As we age there is a
decrease in metabolism,
furthermore,
numerous disease
states involve metabolic
dysfunction (i.e.
ischemia/stroke, cancer).
As all scientists know,
the
major power plant of the cell is
the mitochondria. It
utilizes high energy
intermediates (NADH and FADH) to
donate electrons and drive the
production of ATP, our
functional energy source.
As noted throughout
this website, the most recent
research is focused on finding
compounds that effectively
improve metabolic activity by providing an
alternative electron source
for each cell in the body?
(see graphic to the left)
|
|
Cellular metabolism is critical for
providing the
energy for genes and proteins to be made,
which subsequently influences the
metabolic rate of a cell. Without the
proper levels of energy, errors in gene and
protein transcription can occur.
.
|
.
How important
are Cellular Energy & Mitochondrial
Function?
.
Very Low
energy levels = Possible Genetic
Errors
DISEASE STATE WITHIN THE
CELL
Normal energy levels = Proper Gene &
Protein Transcription
HEALTHY STATE WITHIN THE
CELL
.
|
Metabolic
dysfunction as a result of Mitochondrial
Dysfunction is at the heart of a multitude of
clinical conditions, including cancer. The
approach of implementing products or
compounds that increase or maintain
mitochondrial function thereby combatting metabolic dysfunction can be
viewed as a metabolically targeted therapy
(MTT). |
| |
|
Metabolically Targeted Therapy (MTT)
One example of a metabolically targeted therapy (MTT)
is a new proprietary product line called
Mito~Direct™.
These formulations are designed to transport Electrons(energy),
Vitamins, Minerals
and key Nutrients directly to intracellular
mitochondria.
As is the case with many inferior supplements, if these nutrients are unable to
be absorbed across the intestinal wall and cross the cell
membrane and enter the cell, they can not provide the
body any physiological or nutritional benefit.
This critical delivery system is carried out by
key nutrients that act as a redox polymers. Redox polymers more efficiently accept
and donate charge,
compared to single molecules.
This direct delivery of nutrients and electrons
(directly to mitochondria residing within each
cell) allows
Mito~Direct™
formulations
to serve as an
energy source for
mitochondrial ATP production thereby reversing Hypoxia and Mitochondrial
Dysfunction(see
research below).
|
| |
| |
|
|
| |
| |
 |
|
Has Clinical Research been
looking
in the wrong place
for the past 50 years?
The 3rd party
clinical studies found on this site suggest an emphatic,
.
“YES!”
After reading the 3rd party peer reviewed
articles
listed below and posted throughout this website, one central theme
becomes clear.
.
"Mitochondrial dysfunction has been implicated in
nearly all pathologic and toxicologic conditions."
Experimental and
Molecular Pathology
|
|
|
|
|
|
During hypoxia, the HIF-1 gene
turns on hypoxic dependent genes
and represses or turns off normoxic
dependent genes
The latest research suggests that the
Human Cell contains as many as 20,000
different genes but only a fraction of
these genes are turned on at one time.
THE HEALTHY CELL:
When proper oxygen levels are
available under normoxic
conditions, hypoxic genes, like HIF-1,
are repressed, or turned off
resulting in the homeostasis of a
healthy cell and normal mitochondrial
function.
THE DISEASED CELL:
However, when a cell becomes
hypoxic, HIF-1 levels and other
hypoxic dependent genes are transcribed
resulting in a disease state within the
cell. Increased HIF-1
levels results in the downstream
transcription of Vascular Endothelial
Growth Factor
(VEGF) – a promoter of angiogenesis;
Glucose Transport 1
(GLUT1) and glycolytic enzymes
– critical components in anaerobic
respiration;
and Erythropoietin
(EPO) – responsible for the
differentiation of red blood cells.
(supporting article: Free Radic Biol Med. 2009 Jan)
The transcription
of these hypoxic dependent genes would
have never occurred if the cell had
remained under normoxic conditions.
This cellular response to low oxygen, or
hypoxia, involves the regulation of many
cellular pathways that shut down low
priority cellular activity and increase
stress responses.
These signals from the
environment
during hypoxia activate
various proteins which are called transcription factors.
These proteins bind to
regulatory regions of a gene and
increase or decrease the level of
transcription. By controlling
the level of transcription, this process
can determine the amount of protein
product that is made by a gene at any
given time.
|
| |
| |
QUESTIONS
Why have
scientists spent the last 50 years
trying to repair genes
AFTER
the cell has entered
the disease state
when
the cause of
their dysfunction is now known to be
hypoxia and oxidative stress caused
by mitochondrial dysfunction?
Wouldn't it
make more sense to implement
strategies to prevent hypoxia &
reverse oxidative stress thereby
preventing
the
transcription of each of these
hypoxic dependent genes
that lead to a disease state within
the cell?
Why are these
same scientists suggesting that
disease is based on GENETIC
ERRORS when most of these
errors
occur due to
the transcription of genes that are
only turned on under hypoxic
conditions!
If
mitochondrial dysfunction leads to
oxidative stress & the upregulation
of HIF-1, wouldn't it make sense
to
develop a compound that can reverse
this dysfunction and prevents
hypoxia.
. |
|
|
| |
|
|
| |
|
| Oxidative Stress,
upregulation of HIF-1
and Mitochondrial Dysfunction |
| |
Mo st
causes of mitochondrial dysfunction tend to involve
oxidative stress which can be generated by a myriad
of sources. These levels of oxidative stress can be
dramatically increased and persist at dangerous
levels if the human body is continually exposed to
more than 1 of the following sources at the same
time......see list below.
Exposure to
these sources
can lead to Mitochondrial
Dysfunction |
| . |
alcohol, artificial trans fats,
aspirin, excess calories, glucocorticoids,
homocysteine, iron overload, lipid peroxidation,
lipopolysaccharide, MSG, nutrient deficiencies,
oxidized LDL, pro-inflammatory cytokines,
prescription drugs, sleep deprivation, smoking, statins
and toxic heavy metals.
Per the published studies found on this
website, the human body is constantly exposed to
many of these known causative factors resulting in Oxidative
stress and the upregulation of HIF-1. As
noted in the article below from Johns Hopkins, if this
dysfunction is not reversed, it is
only a matter of time before this long term
oxidative stress will manifest itself in disease and
suffering.
|
.
JOHNS HOPKINS RESEARCHERS PROVE
THAT
HYPOXIC GENETIC SWITCH (HIF-1)
TURNS OFF ATP PRODUCTION
 . . |
|
The Johns Hopkins report
below states that HIF-1 is a
genetic switch that SHUTS
DOWN Mitochondrial function
to protect the cell from
producing ROS which would
result in the overproduction
of Oxygen Radicals. In an
ironic twist, HIF-1 protects the cell from
damage or cell death by
shutting down the whole
system thereby preventing
futher mitochondrial ROS
production.
The formulation goal of
Mito~Direct™ Complexes
is to support HYPOXIC CELLS
and downregulate and/or
attenuate HIF-1
levels. By
reversing HIF-1 levels and
oxidative stress, users can
reverse hypoxia and cellular
dysfunction. Not only are
Mito~Direct™
Complexes
designed to reverse HIF-1
levels, they are also
designed to help to deliver a
constant supply of electrons
directly to the mitochondria
in order to maintain ATP
production. This feature is the
textbook example of how
Mito~Direct™
Complexes
rescue cells from the
damaging effects of hypoxia
associated with HIF-1.
|
|
| |
|
Hopkins
researchers discover unsuspected
genetic switch(HIF-1) that turns off
Mitochondria |
|
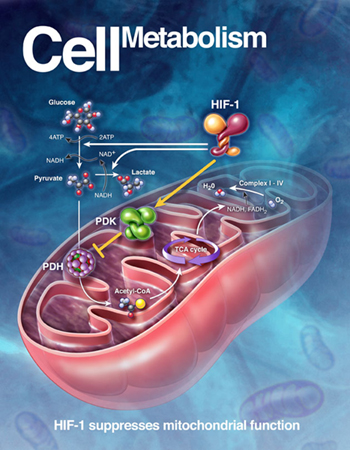
.
HIF-1 suppresses mitochondrial function |
|
A cell’s energy demands are
met by two major types of
sugar ( glucose) using
machines similar to the two
types of engines in a hybrid
car. One machine,
the mitochondrion, is an
organelle that breaks down
the glucose-using oxygen and
produces ATP. The
other does the same thing -
albeit less efficiently -
without using oxygen
in a process called
glycolysis.
Like the hybrid car, cells use
oxygen and the internal
combustion engine at higher
speeds and rely on an electric
engine without need for oxygen
consumption at lower speeds.
Cells consume glucose
through its main
energy-producing machine, the
mitochondrion, when oxygen is
ample. But like the
internal combustion engine, this
process generates pollutants or
toxic oxygen molecules.
At lower oxygen levels, when
cells are starved for oxygen -
as during exertion or trauma --
the genetic switch that
the Hopkins researchers found
deliberately shuts off the
cell’s mitochondrial combustion
engine, which
scientists had long - and
erroneously -- believed ran
down on its own due to lack of
oxygen.
“The unexpected
discovery is that this genetic
switch actively shuts off the
mitochondrion under low oxygen
conditions, apparently to
protect cells from mitochondrial
toxic oxygen pollutants,”
said Chi Van Dang,
M.D., Ph.D., professor of
medicine, cell biology, oncology
and pathology, and vice dean for
research at the Johns Hopkins
University School of Medicine.
Dang says the switch may be a target for
cancer drugs because a cancer cell’s
survival depends on it to convert
glucose to lactic acid through
glycolysis even in the presence of ample
oxygen. Disruption of the
switch(HIF-1) by a drug may cause cancer
cells to pollute themselves with toxic
oxygen molecules and undergo apoptosis
or cell death.
The disruption of this link
blocks the tendency of
the mitochondrion to make toxic
molecules
as it struggles to produce ATP
during hypoxia.
These toxic molecules,
called reactive
oxygen species (ROS),
damage molecules
in the cell and even cause the cell to
undergo apoptosis.
|
|
|
"But our discovery clearly shows that
hypoxia doesn’t simply trigger a passive
shutdown of the mitochondrion,” said
Dang. “Instead, HIF-1 acts as a
genetic switch to actively shut down
mitochondrial function and prevent the
production of reactive oxygen species.”
|
|
|
Free Radic Biol Med. 2009 Jan
Relationship between oxidative stress
and HIF-1 alpha
mRNA during sustained hypoxia in humans.
Abstract
The aim of this study was to investigate
the relations among reactive
oxygen species (ROS),
hypoxia inducible factor (HIF-1
alpha) gene expression,
HIF-1 alpha target gene
erythropoietin (EPO), and
vascular endothelium growth
factor (VEGF) in humans.
Five healthy men (32+/-7 years,
mean+/-SD) were exposed to 12 h of
sustained poikilocapnic hypoxia
(P(ET)O(2)=60 mmHg).
DNA oxidation (8-hydroxy-2'-deoxyguanosine,
8-OHdG), advanced oxidation protein
products (AOPP), EPO, and VEGF were
measured in plasma and HIF-1 alpha mRNA
was assessed in leukocytes before and
after 1, 2, 4, 6, 8, 10, and 12 h of
exposure to hypoxia. HIF-1 alpha mRNA
amount increased during the first two
hours of hypoxic exposure and then
returned to baseline levels. The
findings reveal an up-regulation of
HIF-1 alpha (+68%), VEGF (+46%), and EPO
(+74%). AOPP increased continuously from
4 h (+69%) to 12 h (+216%) of hypoxic
exposure while 8-OHdG increased after 6
h (+78%) and remained elevated until 12
h.
During the "acute" increase
phase of HIF-1 alpha (between 0
and 2 h), 8-OHdG was positively
correlated with HIF-1 alpha
(r=0.55). These findings suggest that
hypoxia induces oxidative stress
via an overgeneration of reactive oxygen
species (ROS).
Finally, this study in humans
corroborates the previous in vitro
findings demonstrating that
ROS
is involved in HIF-1 alpha
transcription.
|
|
|